Medical Product

EuroValve
A normally closed check valve intended for critical infusions that uses a patented sealing mechanism. The product is manufactured from USP Class VI materials and is designed for strength and performance to meet functioning requirements in infusion systems, IV lines, syringes, & diagnostic equipment. Ordering Information
Product No.
0069580-00-00B
Mfg. No.
7003110-005
Description
Low Pressure, Slip Luers
Get a Sample
Quantity
Add to Cart
Add to Cart
+ print friendly version
Materials of Construction:
Filter Material: Silicone Rubber MediumHousing Material: MABS
Biological Safety: USP Class VI Materials
Drug/Fluid Compatibility: Lipids & TPN Solutions
Sterilization Compatibility: Gamma Radiation, EtO
Connections:
Type: TubingInlet: 3mm coaxial Coaxial
Outlet: 3mm coaxial Coaxial
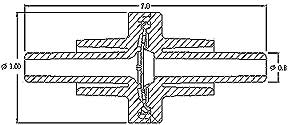
Design:
Overall Dimensions: 0.276" x 0.118" major diameterValve Static Position: Normally Closed
Position Sensitivity: None
IP: Patent Pending
Performance Data:
Minimum flow rate: >/= 200 ml/min @ 1m H20 Head HeightValve opening pressure: <15 cm Wg. (6 in)
Usability: Disposable
Packaging:
Standard Pack: 12,000 parts/carton (bulk, non-sterile)Specifications are for pre-sterilized products and are subject to change without notice. Results in specific applications may vary. Filtertek recommends that end user confirm fitness in specific applications.